CONTENTS
- The Environmental Costs of Hydropower Projects
- Imports Weaken Indian Pharma
The Environmental Costs of Hydropower Projects
Context:
While Wayanad in Kerala is gradually returning to normal after last month’s devastating landslide, a landslide in Sikkim on Tuesday caused damage to six houses and a building belonging to the National Hydroelectric Power Corporation (NHPC) at its Teesta-5 hydropower station in Gangtok.
Relevance:
GS3-
- Environmental Pollution and Degradation
- Disaster Management
Mains Question:
Hydropower projects in the Himalayan region need to factor in the environmental costs too. Discuss in the context of recent disasters effecting hydropower projects. (15 Marks, 250 Words).
India’s Hydroelectric Power Capacity:
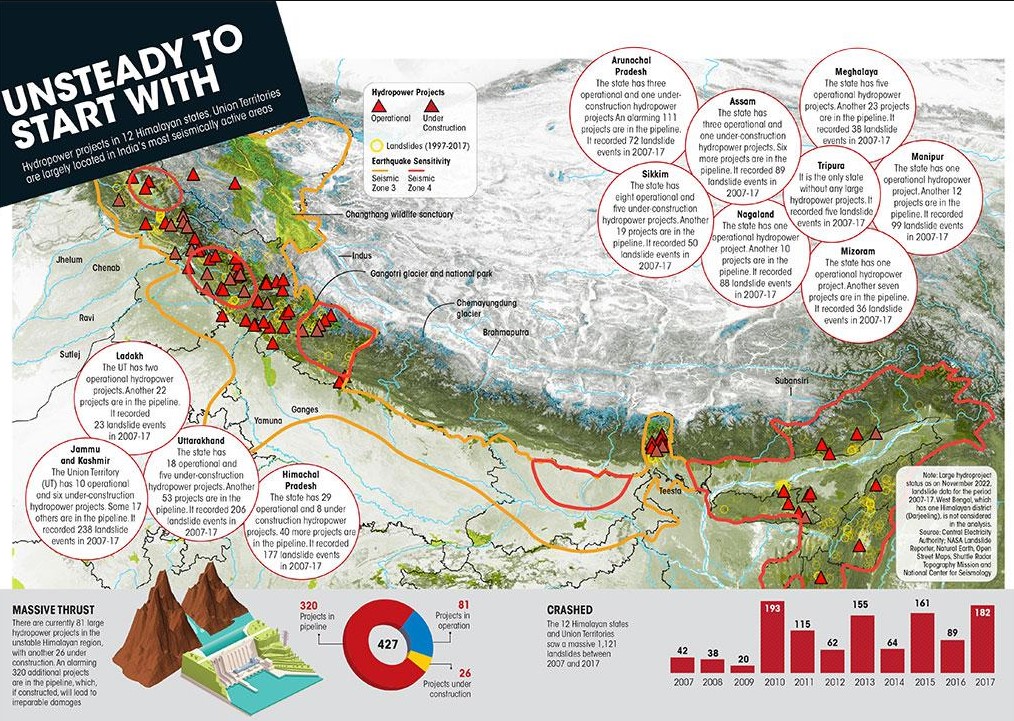
- India ranks 5th in the world for installed hydroelectric power capacity. As of March 31, 2020, the country had an installed utility-scale hydroelectric capacity of 46,000 MW, accounting for 12.3% of its total utility power generation capacity.
- In addition, smaller hydroelectric power units with a combined capacity of 4,683 MW (representing 1.3% of the total utility power generation capacity) have also been installed.
- The Himalayan region, known for its abundant water bodies and favorable topography for electricity generation, is often referred to as India’s powerhouse.
- Government estimates indicate that the region has the potential to generate 115,550 MW of electricity, with an installed capacity currently at 46,850 MW.
- As of November 2022, the region’s 10 states and two Union territories had 81 large hydropower projects (over 25 MW) in operation and 26 projects under construction.
- Additionally, the Central Electricity Authority under the Union Ministry of Power reports that another 320 large projects are in the planning stages.
Disasters Effecting Hydropower Projects:
- Although the impact of the events in both locations cannot be directly compared, as no lives were lost or injuries reported in Sikkim, the situation is concerning.
- This marks the second natural disaster to affect a hydropower project along the Teesta River.
- Last October, a deluge from the South Lhonak glacier in North Sikkim destroyed the Chungthang dam, which was vital to the Teesta-3 power station (not operated by NHPC).
- The Teesta-3 project, which was the largest hydroelectric power project in the state at 1,200 MW, has been largely non-operational since the incident, with only a tenth of its original power output currently available.
- The 510 MW Teesta-5 project has also been rendered non-functional since the glacial lake outburst.
The Debate Surrounding Hydropower Projects:
- The disaster has reignited the ongoing and complex debate surrounding hydropower projects, highlighting the persistent challenges they pose.
- Despite initial plans dating back nearly three decades to establish 47 hydropower projects along the Teesta River in Sikkim and West Bengal, only five have materialized, with around 16 others in various stages of planning and consideration.
- The Teesta, a tributary of the Brahmaputra, originates from Tso Lhamo Lake at an elevation of about 5,280 meters in north Sikkim.
- It flows for approximately 150 kilometers in Sikkim, 123 kilometers in West Bengal, and another 140 kilometers through Bangladesh before emptying into the Bay of Bengal.
- The river’s journey through challenging and varied terrain makes it an attractive prospect for governments seeking to maximize power generation potential.
- However, over the years, the process of developing these projects has been fraught with difficulties. Numerous companies have bid on projects auctioned by state governments, yet the process has rarely been smooth.
- Balancing environmental risks, the costs of adequately mitigating those risks, public perception, and the pursuit of profit has proven to be a complex task.
- For instance, in the case of the Teesta-3 project, reports suggest that developers opted for a concrete-faced rock-fill dam instead of a more robust concrete gravity dam to reduce costs, which may have contributed to its complete destruction.
Conclusion:
This incident underscores the need for thorough and accurate environmental impact assessments for hydropower projects in the region. Such assessments should provide a realistic estimate of the actual costs involved, which is crucial for maintaining public trust in these projects and ensuring their environmental sustainability. By doing so, it would not only enhance public confidence but also promote the long-term viability of these projects in an environmentally responsible manner.
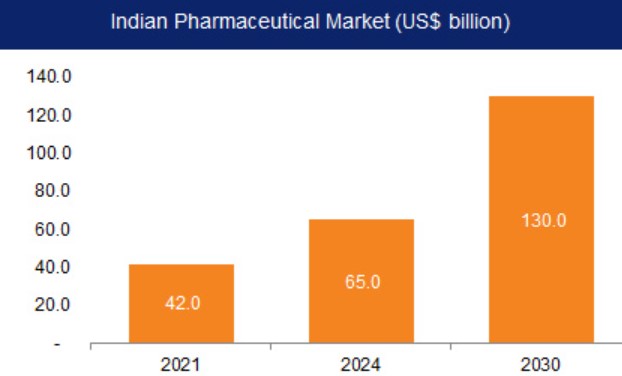
Imports Weaken Indian Pharma
Context:
The government has recently introduced two initiatives that rely on imports to meet domestic needs of medicines, which could have a detrimental impact on the domestic pharmaceutical industry. Although the Drugs Price Control Order of 2013 aims to regulate the prices of existing medicines, a more effective approach would be to foster a competitive environment for essential medicines by encouraging local production.
Relevance:
GS2-
- Government Policies and Interventions
- Issues Relating to Development
GS3- Industrial Growth
Mains Question:
Reliance on imports could have a chilling effect on the pharmaceutical industry, weakening its ability to remain relevant. Analyse in the context of recent initiatives taken by the government. (10 Marks, 150 Words).
Initiatives Undertaken:
Procurement through Global Tenders:
- The first initiative is a Department of Expenditure (DoE) order allowing the Ministry of Health to procure 120 medicines through global tenders for Union government schemes.
- This list includes several top-selling anti-diabetes and anti-cancer drugs, which are currently dominated by a few companies in India due to patent protection, regulatory barriers, or both.
- Additionally, the DoE order specifies a particular brand for over 40 of these medicines, further enhancing the monopoly of foreign companies.
Removing Customs Duty:
- The second initiative, proposed in the 2024-25 Union Budget, involves removing the 10-12% customs duty on three cancer medicines marketed by AstraZeneca, ostensibly to lower their prices.
- However, given the extremely high cost of these medicines, the proposed reduction in import duties would do little to make them more affordable.
Challenges Associated with These Measures:
- These measures risk disincentivizing domestic producers and increasing the country’s reliance on imports.
- More critically, they could reinforce two significant entry barriers faced by the domestic industry: the product patent regime and the stringent regulatory guidelines for marketing bio-therapeutics.
- New medicines are typically under patent protection, which prevents Indian companies from producing affordable generics or biosimilars.
- Meanwhile, the regulatory guidelines impose expensive and time-consuming requirements for obtaining marketing approval for biosimilars, potentially harming domestic producers.
Way Forward:
- These entry barriers can be addressed through proactive government intervention. The Patents Act contains several public interest provisions that can be invoked to promote local production.
- Similarly, the regulatory guidelines for marketing bio-therapeutics can be revised to ease the burden on domestic companies.
- Section 83 of the Patents Act emphasizes that patents are granted not just to encourage inventions, but also to ensure that these inventions are commercially utilized within India to the fullest extent possible without undue delay.
- The Act specifies that patents should not simply enable patentees to monopolize the importation of the patented item.
- It also mandates that patents should make the benefits of the invention available to the public at reasonably affordable prices.
- Substantive provisions within the Act uphold these principles, ensuring that while patent holders are entitled to their rights, they must not act in ways that harm the public interest.
- If a patented medicine is not available to the public at a reasonably affordable price, compulsory licenses (CL) can be issued to any company willing to produce the product in India.
- CLs are an effective tool to ensure the affordability of medicines, though they have been used only once when an originator company priced a medicine at nearly ₹3 lakh.
- An Indian company, using a CL, was able to produce it for ₹8,000. Despite the high cost of many medicines, the Patent Office has not issued any other CLs, even during the COVID-19 pandemic.
- This is in stark contrast to the U.S. government, which granted licenses on multiple patents during the pandemic.
- India’s Patents Act also allows for the granting of government-use licenses under Section 100, which states that patents do not restrict the Central government from taking measures to protect public health.
- This section permits the government to issue licenses to facilitate the domestic production of generic versions of patented medicines.
- In addition, the guidelines for marketing approval of biosimilars in India are outdated and resource-intensive.
- For example, current guidelines mandate animal studies, which are no longer required in developed countries with strict regulatory standards, such as the U.S. and the EU.
- The World Health Organization (WHO) and U.K. guidelines treat clinical trial requirements as exceptions rather than rules, whereas Indian guidelines still mandate clinical trials. These outdated requirements create further obstacles for Indian producers.
- The International Generic and Biosimilar Medicines Association recently highlighted that eliminating these redundant requirements could significantly improve patient access by saving time and resources.
- The proposed duty waiver on cancer medicines and the global tendering for critical medicines contradict Parliament’s intentions to enhance access and affordability of medicines through domestic production, as outlined in the Patents Act.
Conclusion:
Relying on imports could stifle the domestic pharmaceutical industry, diminishing its relevance. The government needs to reassess these recent decisions and, more importantly, align its policies to support the growth of the domestic pharmaceutical sector. Ensuring the affordability of pharmaceuticals is crucial for managing healthcare costs, particularly in India, where out-of-pocket health expenses made up nearly 47.1% of the total health expenditure in the year 2021.





